Search
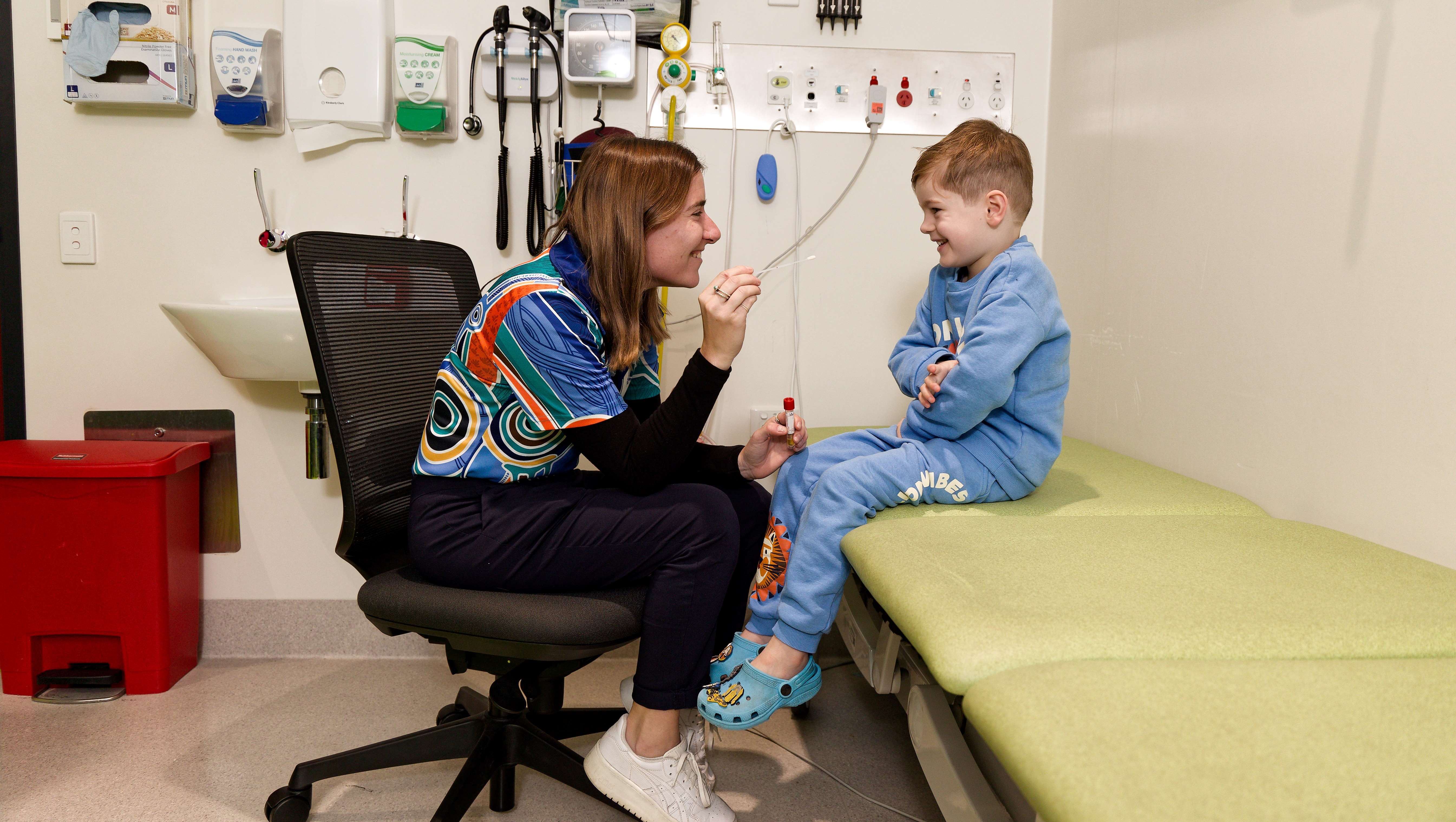
Researchers from The Kids Research Institute Australia would like to understand more about respiratory syncytial virus (RSV) and how we can provide the best protection for kids.
Review the hospital-based research that the Wesfamers Centre of Vaccines & Infectious Diseases conducts.
We are evaluating new vaccines for a range of diseases including influenza, pneumococcal, meningococcal and common infections such as otitis media (glue ear).
Vaccine Trials Group with Sir Charles Gairdner Hospital is conducting a trial of a vaccine against Clostridium difficile infection (CDI) in at-risk individuals.
The study aims to determine whether an RSV vaccine given to pregnant women during the third trimester can protect newborn babies from RSV infections.

Through co-design with community members, we hope to better understand the strengths and effectiveness of community-driven health promotion resources.
The aim of the study is the early identification of problems with the current flu vaccines, and providing parents and professionals with up to date information.
The main objectives were to evaluate effectiveness of the annual flu vaccine in young children, and the burden of flu on young children and their families.
The PAEDS Study monitors childhood conditions of public health importance that are difficult to effectively capture through other surveillance mechanisms.

Researchers at The Kids Research Institute Australia are studying a new pneumococcal vaccine designed to provide a broader protection for 21 serotypes of the bacteria S. pneumoniae – 8 more serotypes than the current vaccine given to new babies.
